Emovi is a medical technology company and creators of a device called the KneeKG™, the first in-clinic device for measuring knee function with objective and quantifiable data. Developed in partnership with the Quebec university research community, the KneeKG™ provides functional information to healthcare professionals with a higher correlation to symptoms and at a fraction of the cost of high-tech scans such as MRIs. It allows doctors to significantly improve patients’ quality of life, pain and limitations. Emovi owns multiple patents and the exclusive license to commercialize the KneeKG™ worldwide, and is revolutionizing the way knee assessments are performed.
Challenge
From 2012 to 2017, Emovi focused its pre-commercialization efforts on testing and publishing results on the efficacy of the KneeKG™ by bringing the technology to various academic centers and research teams. Despite the early success of these tests, getting KneeKG™ to market was not without its challenges. From EMR integrations to HIPAA compliance, to building a complete care delivery solution, forming the right partnerships was a critical step in their journey. “We found that good and dynamic partners such as MedStack are very important,” says President & CEO Michelle Laflamme. “For medical devices, getting from research to the hands of clinicians is quite a challenge because we are introducing a new way of assessing the patient. It is amazing to collaborate on delivering a complete solution that impacts all areas of care and have it move smoothly into implementation.
Solution
Emovi was an early MedStack customer, first creating harmonized production and staging environments on MedStack’s legacy product, MedStack Classic. Working in partnership with app development company SiteRocket Labs, the result was a fully compliant cloud hosting environment that empowered Emovi to efficiently manage their applications on production while mitigating the risk of service interruption. MedStack’s out-of-the-box platform included provisioning for a VPC and intrusion detection system to harden application infrastructure security, as well as a backup system to capture redundant copies of hourly, daily, weekly, and monthly changes impacting data and infrastructure configurations. MedStack’s solution scaled to support Emovi’s expansion into the UK, and since then Emovi has also created and deployed additional environments on MedStack Control.
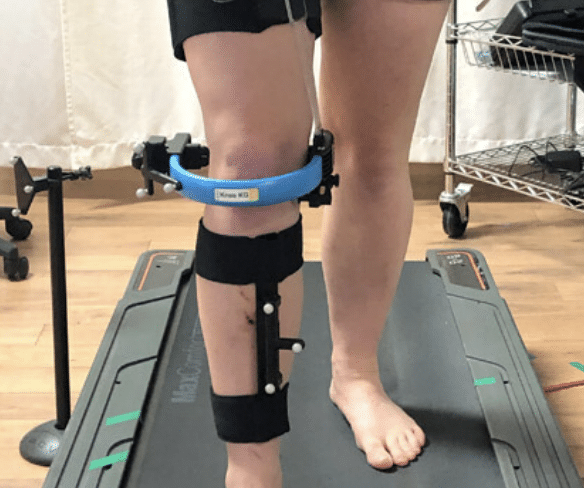
Impact
MedStack’s turnkey compliance solution enabled Emovi to quickly deploy a HIPAA-compliant application and begin selling into multiple jurisdictions. This helped to attract investor attention and propelled the company into a stage of high growth. In March 2019 Emovi successfully raised a $15M Series C round.


